Paul Bonvallet, Department of Chemistry
Swellable organically modified silica (SOMS) is a hybrid organic/inorganic media discovered at the College of Wooster. Sometimes called “swellable glass,” this hydrophobic material absorbs many times its mass in organic liquids like acetone (Figure 1). The reversible swelling behavior has applications ranging from personal care products to environmental remediation. However, the fundamental chemical and physical principles that govern its behavior are poorly understood.
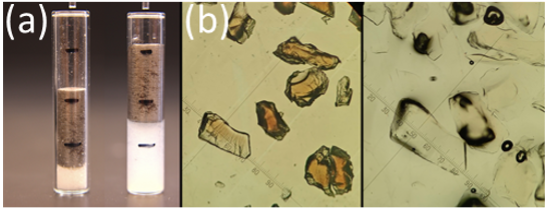
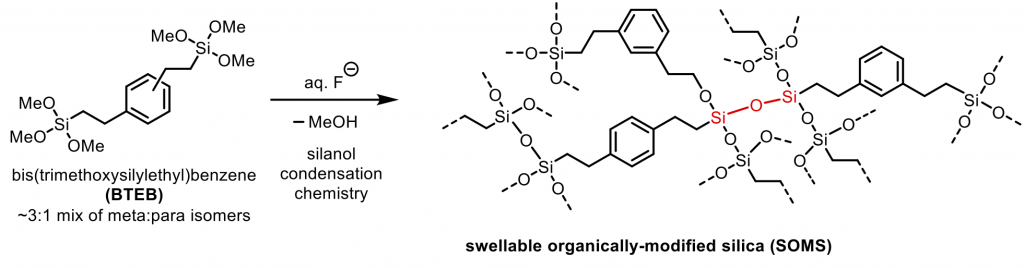
- Chemical Formulation Project. SOMS is prepared from a silane precursor called BTEB. Under fluoride-catalyzed conditions, the silane is converted into a silanol, which then undergoes condensation in three dimensions to form a silane (Figure 1). We are interested in learning how monomer structure, reaction conditions, and degree of polymerization are related to the mechanical properties of the material.
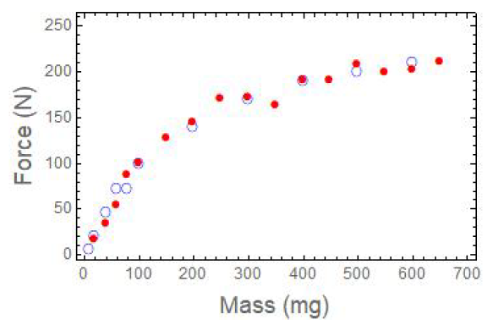
- Force Measurement Project. SOMS generates a mechanical force when it absorbs organic liquids and swells. We have an apparatus that measures this force, and we have made some interesting discoveries. Loading the device with more SOMS generates a greater swelling force (up to several hundred Newtons), but not linearly (Figure 2). We have developed a theoretical model that describes the differential force generated by a sample of SOMS as a function of its intrinsic modulus and the physical dimensions of the space that it occupies. We are currently investigating environmental factors that influence the swelling force (temperature, type of solvent).
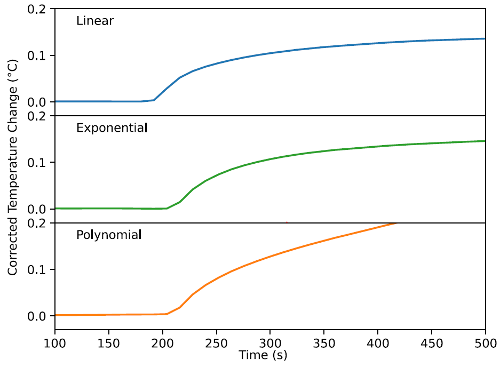
- Calorimetry / Thermodynamics Project. The swelling of SOMS is spontaneous at room temperature, but little is known about the basic thermodynamics of this process. Calorimetry experiments provide insight on the exchange of thermal energy when SOMS swells (Figure 3), which leads into foundational knowledge about enthalpy change. We can build upon this information to learn about entropy change and free energy change.
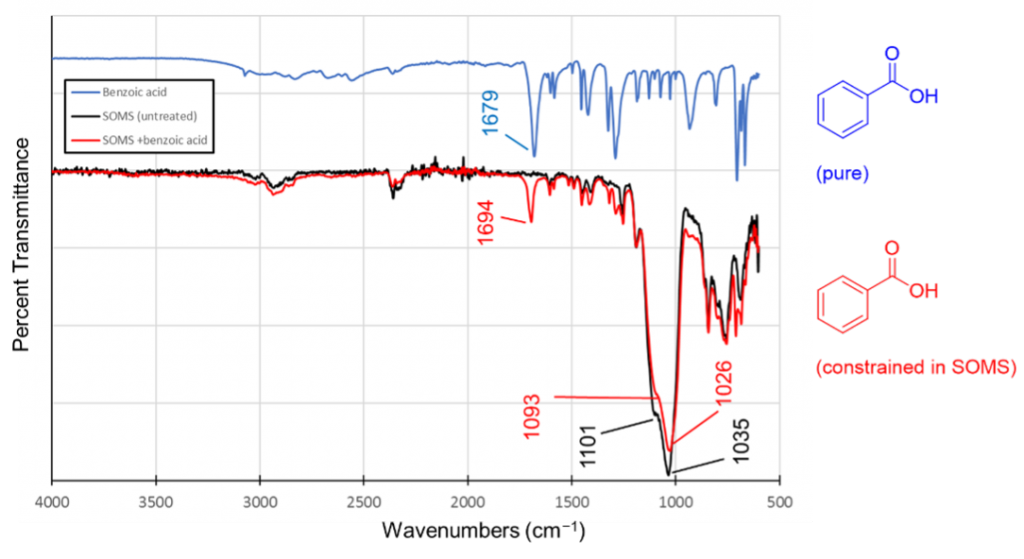
- IR Spectroscopy Project. We can “load” SOMS by treating it with solutions of organic compounds in organic solvents. Evaporating the solvent leaves the solute behind, trapping it inside the SOMS matrix. Carbonyl-containing compounds exhibit differences in their vibrational (IR) spectrum when trapped inside SOMS. Interestingly, the carbonyl stretching frequency of numerous carboxylic acids, aldehydes, and ketones increases by ~15 cm−1 when the compound is trapped inside SOMS in comparison to the pure compound (Figure 4). Similarly, the emission wavelength of certain fluorescent materials changes inside SOMS. We are exploring the physical and chemical basis for these observations using IR and fluorescence spectroscopy.
We will collaboratively design experiments in the areas described above. Curiosity, organization, and attention to detail are more essential than prior experience with advanced chemistry.
